In August 2024, the FDA approves a groundbreaking blood test for colon cancer that can screen colon cancer, offering a less invasive and more convenient alternative to traditional screening methods like colonoscopies. This newly FDA-approved blood test for colon cancer screening has the potential to revolutionize the early detection of colon cancer, especially for individuals who may have avoided more invasive tests. For Connecticut residents, this new advancement offers hope in the battle against colon cancer, which continues to affect many across the state.
How Does the FDA-Approved Blood Test for Colon Cancer Work?
The FDA-approved blood test for colon cancer works by detecting specific biomarkers in the blood that are linked to cancerous growths in the colon. This cutting-edge technology is designed to find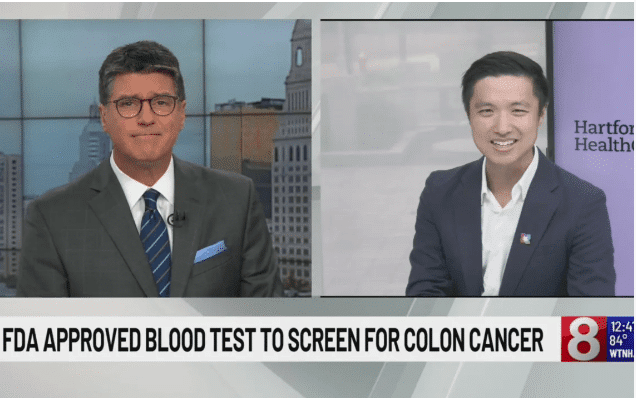
Why Is Early Detection of Colon Cancer Important?
Early detection is the key to successfully treating colon cancer, which is one of the most common forms of cancer in the United States. When caught in its early stages, colon cancer is highly treatable, but unfortunately, many individuals delay or avoid screening due to the invasive nature of colonoscopies. This new blood test provides an option that is easier for patients to access, which could lead to increased screening rates and a reduction in late-stage diagnoses. Dr. Tai Ho, a Gastroenterologist with PACT – Hartford HealthCare, emphasized in a recent interview with News 8 how critical early detection is for saving lives, and how this new blood test can help achieve that goal.
Watch the full interview- HERE.
The Impact of the FDA-Approved Blood Test for Colon Cancer Connecticut Residents
Colon cancer rates have been on the rise in Connecticut, and this new blood test represents a crucial advancement for those at risk. According to Dr. Ho, this test could have a significant impact on increasing early detection in the state, especially for individuals who may have previously avoided screenings. For Connecticut residents, this test offers a new level of accessibility and convenience, making it easier to prioritize health and catch colon cancer before it becomes a more serious issue.
With the approval of this blood test, Connecticut residents now have an additional option for colon cancer screening, and with its simplicity and accuracy, it is expected to improve health outcomes statewide.
Who Should Consider Getting the Blood Test for Colon Cancer?
The blood test for colon cancer is recommended for individuals who meet certain criteria, including:
- Adults aged 45 and older
- Those with a family history of colon cancer
- Individuals with a history of polyps or gastrointestinal issues
- Anyone who may have avoided colonoscopies due to fear, discomfort, or lack of access
If you fall into one of these categories, it’s important to discuss this new screening option with a gastroenterologist to determine if it’s right for you. The earlier colon cancer is detected, the more treatment options are available, and the better the chances of a full recovery.
Take Action: Schedule Your Blood Test Screening with PACT Gastroenterology Today
If you or a loved one are over the age of 45 or have risk factors for colon cancer, now is the perfect time to take advantage of this FDA-approved blood test. Early detection could make all the difference in your health and future, and this new test makes screening easier than ever.
Contact PACT Gastroenterology today to schedule your colon cancer screening consultation and find out more about how this new blood test can benefit you. Don’t delay—protect your health and ensure peace of mind with this revolutionary screening option.
FAQs About the FDA-Approved Blood Test for Colon Cancer
- Is the blood test as accurate as a colonoscopy? While a colonoscopy remains the gold standard for colon cancer screening, the FDA approves blood test for colon cancer screening will allow providers to have an alternative highly accurate detection in early-stage colon cancer through biomarkers in the blood. It provides a less invasive option for those who may not be able to undergo a colonoscopy.
- How often should I get the blood test for colon cancer? It’s recommended that individuals over 45 get screened regularly for colon cancer, but the frequency of blood tests should be discussed with your healthcare provider based on your specific risk factors and medical history.
- How soon will I get the results from the blood test? Results from the blood test can often be obtained within a few days, providing quick insights into your health and allowing you to take any necessary next steps promptly.
- Does insurance cover the FDA-approved blood test for colon cancer? Most insurance providers are beginning to cover this new blood test as part of standard colon cancer screenings. However, it’s best to consult with your insurance company to confirm coverage.
Contact PACT Gastroenterology to Learn More About the FDA-Approved Blood Test for Colon Cancer
Connect with PACT Gastroenterology today to learn how the FDA-approved blood test for colon cancer can help you take control of your health. Early detection is vital, and with this innovative test, you can protect yourself from the risks of colon cancer with greater ease and convenience than ever before. Ready to schedule your screening? Find a provider today!